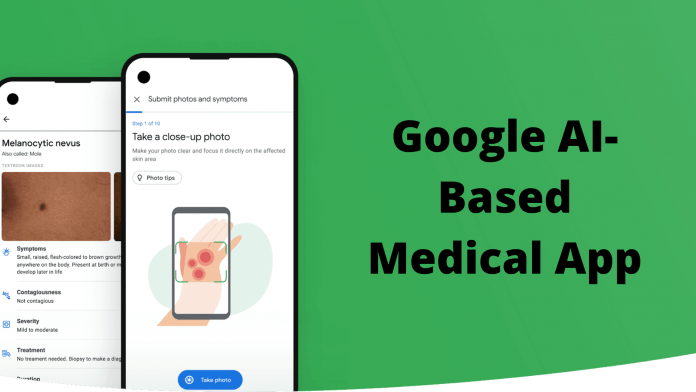
Billions of times each year, people use Google’s web search window to figure out what’s wrong with their skin. Now Google is preparing to release an app that uses image recognition algorithms to provide more professionally customized and help. A brief demo at the company’s developer conference last month showed a service that offers some possible skin conditions based on uploaded photos.
Algorithms and research in which doctors observe burns in patients in the past are comparable to or even surpassing mechanical dermatology. However, there is little evidence from clinical trials of adopting these technologies, and the AI image analysis tools used by dermatologists in the United States are not approved, says RoxanaDaneshjou, a machine learning and health researcher at Stanford University’s Department of Dermatology. “A lot of people don’t work in real-world environments,” she says.
Google’s new app hasn’t been clinically validated yet, but the AI dermatology app is getting a lot of attention because of its AI capabilities and recent health care department builds. Nevertheless, skin services are launched on a small scale. The largest market in the United States is far from home. This service is not analyzing US skin scars in the near future.
At a developer conference, Google’s chief health officer Karen told Karen DeSalvo that he plans to announce that the company will call it a dermatological aid tool in the European Union later this year. The video in this app suggests that the trace of someone’s arm is the point with the caption of an EU-approved medical device. A warning “Not available in the US” was added to the same note.
The company’s America not first strategy emphasizes how easy it is to get approval for a medical app in Europe than in the United States. A Google spokeswoman said there was no timeline for crossing the Atlantic when the company wanted to serve in the United States. They declined to mention whether Google had discussed the app with the U.S. Food and Drug Administration but acknowledged that the FDA’s approval process was longer.
Google says the skin app is “CE marked as a Class I medical device in the EU”. This means it can be sold in blocks and other countries that recognize that standard. HughHarvey, managing director at UK digital health consulting firm HardianHealth, says relatively few obstacles are faced to ensure this clearance. “Basically, you just fill out a form to prove yourself,” he says. Last month, Google’s meeting was held a week before the EU’s strict rules came into force. According to Harvey, many health apps, including Google’s, are needed to show that the app is effective. Existing applications must comply with the new rules by 2025.
Last month’s demo was easy App design isn’t final AI health software U.S. experts may face the FDA’s more complex process when Google brings skin apps home I say I can. An FDA spokeswoman declined to comment on Google’s services, but software that claims to be used for “diagnosing, treating, preventing or treating a person” can be considered a medical device and is an institution. Said that approval could be required. A spokesman said that in order to make this call, one generally had to “consider the intended use of the software and product claims.” A spokesman added that the agency has published guidelines to encourage data collection for diverse populations.
In the design shown in the demo, a person takes three pictures of a scratch at other angles and distances. Users can selectively add information about the affected body part and how long the problem occurred. Tap “Send” to compress the image to Google. Next, the app “Recommended conditions” is displayed, and possible conditions are displayed in the image. Tap any of the symptoms to see a list of important information such as infection and treatment options. Google has trained the app on “hundreds of thousands of skin images” to identify 288 conditions, including skin cancer, accounting for about 90% of common dermatologist web searches.
With medical device approval, the FDA exempts some health software that is considered “low risk”, such as “happiness” advice such as diabetes management and information on health symptoms. Requires the approval of someone who provides a particular diagnosis or someone else, such as an app that runs on a medical device such as a stethoscope. The boundaries between apps that require permission and those that don’t are difficult to find exactly because the medical software and the rules for managing it are relatively new.
Epstein BeckerGreen regulatory attorney Bradley Thompson asks clients some important questions when determining whether FDA approval is required. This includes how the output of the software will be presented to individuals and whether the company will make certain medical claims.
The Google app does not highlight the condition of the skin as much as possible depending on the portrait and warns “The conditions listed here are not recommended for medical diagnosis” A company spokesperson likened this application to a search engine and displayed the results for a person to peruse and draw their own conclusions.
But Google also emphasized medical cutting of skin apps. Health officer De Salvo said Google developed the app because there aren’t enough skin specialists to help everyone with skin disorders. A Google blog post linked the app to a peer-reviewed study comparing the company’s technology with doctors: “Our AI system can be as accurate as an American-certified dermatologist.” Said.
It caught the attention of the proud Thompson lawyer. “It’s really nice that this is at least the same as having a human doctor,” he says – the type of claim the FDA might be interested in.
Daneshjou, a dermatologist and researcher at Stanford University, believes that Google applications can appear to consumers and regulators as if they were providing medical expertise as well as search results. The application may be considered a “high-risk” device because some skin conditions, such as melanoma, can be dangerous, she says and requires FDA approval.
Google also has to publish details about how it tested the technology on different skin tones, Daneshjou said. Until now, the company’s research on artificial intelligence dermatology has shown that relatively few people have dark skin.
Google faces a real challenge when deploying other promising AI health software out of the lab. In 2018, the company began testing a system capable of detecting eye diseases in diabetic patients in hospitals in Thailand. In 2020, the company published an investigation into a rollout that found that the system rejected more than 20% of images of patients due to issues such as variable lighting and practical constraints for nurses.
Read about the Best Learning Apps on Google Play Store